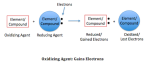
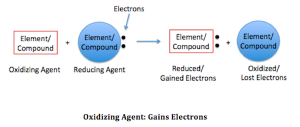
 Reduction reaction is a chemical reaction where there is a reduction of oxidation number. The substance that undergoes the reduction reaction is called oxidizing agents.
Reduction reaction is a chemical reaction where there is a reduction of oxidation number. The substance that undergoes the reduction reaction is called oxidizing agents.
An oxidizing chemical material is a chemical material that is not easily flammable but producing oxygen that can cause fire. Other than getting a fire, these chemicals can also be one reason why firefighters having difficulty to extinguish fire.
In general, oxidizing chemical materials could cause these effects:
- Oxidizing chemicals can either be poisonous and corrosive.
- Speed up the inducing of fire.
- These materials caused non-flammable materials can easily got into fire rapidly.
- These materials could cause a flammable material got into fire spontaneously with no clear source of flaming material around.
- Oxidizing materials are non-flammable material, but it could cause fire easily. Burning involves an oxidation from a flammable compound. When a flammable compound caught a fire, there is a chemical reaction where the flammable compound bonded with an oxygen. Then produced heat, gas, even burning fire. Oxidizing compounds could supply oxygens and giving support to fire even when there is no air around. During producing an oxygen, there is a kind of oxidizing agent that needed heat for the process and there is also the kind of oxidizing agent that only needs a room temperature.
- Impact of contact with an oxidizing agent with a flammable compound depends on the stability of the oxidizing compounds itself. The more stable it gets, the reaction that is caused would be more dangerous.
Thus, that’s all the effects of oxidizing chemicals. You may also read about Oxidizing Material Effects for Health and Environment
These are some oxidizing chemicals which can cause some side effects:
- Ozone (O3)
Ozone is a chemical compound that is classified as a strong oxidizing agent. Ozone can be found in the earth atmosphere. In the industrial sectors, ozone is already used as a solution to reduce water pollution, clothes cleaner and bleach, preserve foods, and much more.
Ozone can also be used in the medical sectors as it is used in the ozone therapy. Despite having many benefits, ozone’s trait as a strong oxidizing chemical also cause a major side effects, especially if the therapy isn’t done accurately. Often, there’s a complaint from a post-therapy patient, like;
- Dizziness
- Respiratory system disorders (shortness of breath or dyspnea)
- Pain in the chest part
Therefore, to prevent those side effects, we must make sure that the therapy procedure is done by a licensed institution. The given dosage must be doublechecked and according to ISCO, that is not more than 200ml of the patient’s blood. Bear in mind that this therapy can’t be done to a pregnant woman, and not recommended for a woman on a menstruation. You may also read about Common Chemical Oxidizing Agents
- Hydrogen peroxide (H2O2)
The hydrogen peroxide compound is mainly used as a composition for various products, like bleach, detergent, antiseptic, and many more.
But, usage of hydrogen peroxide without concern or using it more that the recommended dosage could cause harm to the body and even the nature. These are the side effects that can be caused by hydrogen peroxide;
- Hydrogen peroxide could cause wider burns on your skin if applied to treat a serious and deep burns.
- Hydrogen peroxide causes and speeds up fire and explosions especially if there is a friction, heat, or contamination.
- Contacts with skins or eyes could cause serious burns or cornea perforation.
- Inhaling or swallowing hydrogen peroxide with a 10% or higher concentrate could cause ulcers, allergy, swelling in the mucous membrane, etc.
You may also read about Hydrogen Peroxide Applications
- Ammonium perchlorate (NH4ClO4)
This chemical material is a mix between strong oxidizing agents (perchlorate) and a good fuel (ammonium). Both explains how ammonium perchlorate can be used as a rocket fuel.
Ammonium perchlorate is a chemical compound that is used as an exploding materials and fireworks, as a rocket oxidizing agent, glue, etc. This chemical compound can be unraveled forming a poisonous gas like chlorine, Chloride acid, Nitrogen oxide in a high temperature. This compound can make a huge reaction with a flammable material, reducing agents, and metals.
Health wise concern, contacts with this compound could cause irritation in the eye and skin.
Storage and Usage
Storage of this oxidizing chemical compound can’t be done carelessly, because this chemical compound is considered hazardous. There is some standardized operational procedure to store these oxidizing chemicals, so that it is no longer hazardous while in store.
Moreover, in usage, there is a must on understanding how to use this chemical compound correctly so that we can use it safely. Storage and usage procedure this oxidizing compound explained as below;
- Store it in a cold temperature, ventilated, dry storage room
- Keep the storage room temperature around 14°C, avoid storing with temperature more than 49°C and avoid direct sunlight
- Avoid heat and fire source
- Avoid it from reducing chemical agent or any flammable material
- Store it in a fireproof building
- Store it away from flammable, combustible, and reducing agents like Zn, formic acid (HCOOH), and alkali metals
- Store in a container that’s not made of wood
- Store it where there are tools to clean its residue
- Give a clear label, with an accurate information concerning the usage or storage of this compound.
- Make a scheduled inspection to make sure there is no leaked or opened container
- Make sure that the container closed tightly to prevent leaking or contamination after the usage
- Wash your hand thoroughly after working with these chemicals
- Avoid contact with nose or rubbing eyes with hands that are contaminated by these oxidizing chemicals
- When handling this strong oxidizing agent, use standard laboratory equipment like, shoes, trousers, lab jacket, lab goggles, and gloves
- When doing an experiment regarding mixing these oxidizing compounds with another compound, learn necessary information beforehand about the possible chemical reaction. So that, explosions or such can be prevented
- Residue of this compound after usage should not be put back to the container. Contamination from other chemicals can cause a dangerous reaction.
Act of Prevention
We can’t deny that even if we already work with those oxidizing chemicals carefully, we can’t fully avoid accidents that might be very dangerous. Accidents still might happen. Therefore, we need to know a preventive act as a first aid when there might be accidents;
- Skin contact; Wash the skin that is contaminated by the compound using water about 15 minutes until the pain go down. if it continues or the chemicals is poisonous, ask for medical aid.
- Eye contact: Rinse your eye with water for about 15 minutes, pull your upper and lower eyelid gently then spin your eyes around.
- Inhaled: breath fresh air immediately.
- Swallowed: Rinse your mouth with water, avoid provoking vomit.
- Leaking: For a solid oxidizing compound, sweep and collect it. For a liquid compound, clean it with an inert absorbing sponge. If the liquid compound is acidic, neutralized it with sodium bicarbonate (NaHCO3)
You may also read about:
- 5 Harmful Irritant Chemicals Examples
- 15 Harmful Effects of Chemicals on Biodiversity
To sum up, there are some harmful effects of oxidizing chemicals. So, we need to be careful and minimize those bad chemicals effects.