 What’s the monatomic gas definition? Monatomic compounds were short form of mono (one) and atomic (atom). Therefore, you can consider monatomic compound as a single atom. Monatomic is different from diatomic where as diatomic means that it consists of two different atoms. When there’s a single independent atom, it is considered as a monatomic.
What’s the monatomic gas definition? Monatomic compounds were short form of mono (one) and atomic (atom). Therefore, you can consider monatomic compound as a single atom. Monatomic is different from diatomic where as diatomic means that it consists of two different atoms. When there’s a single independent atom, it is considered as a monatomic.
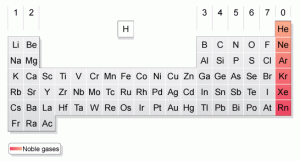
You considered it as monatomic because that atom stands on its own and it is in its purest state. Take the example of the VIII A group in the periodic table that has an electron stability on its electron octet. Therefore, the noble gas is referred as stable in its monatomic state. The VIII A group of elements in the periodic table consists of He (Helium), Ne (Neon), Ar (Argon), Kr (Krypton), Xe (Xenon), and Rn (Radon).
Monatomic and Diatomic Gas Side by Side
- Monatomic gas has one atom whereas diatomic gas has two atoms
- Monatomic gas is generally not stable whereas diatomic gas is generally stable
- There is a chemical bond between diatomic elements but there is no chemical bond in a monatomic element
Noble Gas
Noble gasses are the VIII A group of elements in the periodic table as seen by their order in the table. They were considered as the VIII A group of elements because noble gasses are the most stable gas. This unique trait of noble gas made them very hard to react or inert with another element.
Looking by their outer orbital electron configuration, elements in the noble gas groups are fully configured. Every element in the noble gas group is octet, except for Helium which is duplet.
Characteristics and traits of noble gasses:
- Noble gas is a colorless, tasteless, and scentless kind of gas
- Noble gas has a very low freezing point and boiling point compared to other groups of elements in the periodic table. The noble gas attractiveness between particles in its fluid phase is the same as its attractiveness between particles in the gas form. It is because the noble gas has a low freezing and boiling point.
- The atomic radius of a noble gas is very small, its ionization energy is very high with a very small value of electron affinity. That characteristic of a noble gas states that it is very hard for noble gas to release or even accept an electron. Therefore, noble gas has an inert trait.
- Noble gas can only be found in a monatomic state and can’t be found in diatomic state.
- Noble gas has a very stable state because it has 8 electrons in its outer orbital layer.
- It is very hard to find noble gas compounds in nature because of its monatomic characteristics.
According to Lord Rayleigh and Sir William Ramsay, you can find Argon gas in the nature specifically in a humid air along with Nitrogen and Oxygen.
These are the abundance number of noble gasses you find in the air:
| Unsur | Volume di udara (%) |
| Helium | 0.000524 |
| Neon | 0.00182 |
| Argon | 0.935 |
| Kripton | 0.00112 |
| Xenon | 0.00000088 |
| Radon | – |
You may also read about List of Chemicals on the Periodic Table
Ion Monoatomik
History of Noble Gas
History of noble gas started in the year 1785 following Cavendish’ discovery. Cavendish found that about 1/2000 portion in the air totally has no reaction even though the reaction already involves gasses in the atmosphere. In 1894, Lord Rayleigh and Sir William Ramsay successfully separated one of the gas elements in the atmosphere (which today known as noble gas) according to data spectrum.
Then they tried to react that gas with another element, but they failed. The gas was later named Argon. In the following year, Ramsay successfully isolated Helium. This was due to Janssen discovery in 1868 during a total solar eclipse.
Janssen discovered the Helium spectrum from sunlight in the form of yellow line. The name Helium itself is suggested by Lockyer and Frankland. In 1898, Ramsay and Travers got new elements such as Krypton, Xenon, and Neon. Krypton and Xenon was found in the residue of a humid air that almost completely evaporated. In other hand, Neon was found by liquifying the air. In 1900, Radon was found by Friedrich Ernst Dorn, which he referred as radium emission. The name “Radon” itself was not introduced until 1923. You may also read about Examples of Monatomic and Diatomic Gases
Monatomic Ion
Monatomic can also be considered as a single atomic ion. For example, the Potassium ion which is a cation and the Chlorine ion which is an anion. The forming bond between that cation and anion works with the principle of the releasing and accepting an electron.
In a neutral atom, the number of its electron and proton is balanced. Ion which accepts, or releases electron can either produce positive electrical current or negative electrical current. Ionic compounds like NaCl, KCl, and NaOH in its solvent will be oxidized into monatomic ions.
Ion can also be formed through a melting process of a compound using an X-rays, gamma rays, or even radiation. When that compounds easily oxidized into water, then that compound can be considered as an electrolyte solution. Take the example of salt water or any water that contained NaCl compound will easily carry out electrical current compared to distilled water because it is helped by the electrons inside those solutions.
Take another example of an H+ ion. When there’s a H+ ion which is an acidic ion reacts with an OH- ion which is a basic ion, they would able to form water (H2O) along with the residue ions will form salt. In a precipitation reaction, the ion itself does not involved in the fixed sediment.
More Chemistry Topic Here:
- Drawbacks of Democritus Atomic Theory
- Advantages and Disadvantages of Dalton’s Atomic Theory
- Atomic Theory by Rutherford
Ideal Internal Energy of Monatomic Gas
You can also calculate the internal energy (ΔU) of a monatomic gas which is a process of a state of a system that is altered. But you can only observe the initial and the final state of the system itself.
The formula of calculating the value of monatomic gas internal energy will be explained below:
ΔU = 3/2 nRΔT
Whereas:
ΔU = energy differences
n = number of molecules
R = gas constant 8,31 J/Mol
ΔT = differences of the initial and final temperature
Thereby our explanation about the monatomic gas definition. Hopefully this article can be useful for the readers.

