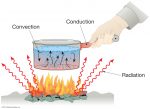
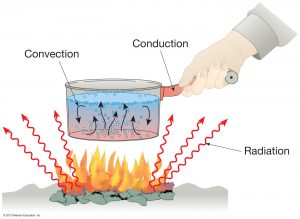
 Based on its medium, the heat transfer process consists of three types and conduction is one of them. Conduction is the transfer of heat through an intermediate medium of substances, but only the energy of it without accompanied by the media transfer.
Based on its medium, the heat transfer process consists of three types and conduction is one of them. Conduction is the transfer of heat through an intermediate medium of substances, but only the energy of it without accompanied by the media transfer.
The heat flow speed is affected by the temperature difference between the delivery ends (ΔT), the area of the conducting cross section (A), the tip spacing having the temperature difference (L), and the type of conductor.
Mathematically can be written with the following formula:
H = Q / t = k. A. ΔT / L
Information:
H : calories that propagate unity of time (J / s or watts)
Q : calor (J or kal)
k : conduction coefficient (thermal conductivity) (J / msK or W / mK)
t : time (s)
A : cross-sectional area (m2)
L : metal length (m)
Q : Temperature (K)
Conducting Heat Transfer Example
Objects that are conductive or able to transfer heat well are called conductors. Lots of tools are created by utilizing conduction events with a tool that is conductor. Here is the heat transfer through conduction example in daily life.
- The fused iron end to make a traditional weapon will be spread evenly to the unheated part.
- Wok in the heat when cooking.
- The lid used to cover the pan will heat up when it is heated
- Spoon or fork will come hot when we used it to stir a very hot food
- A hot iron when the power is on
- The oven is heated when baking the cake.
- The exhaust will heat up when the motor engine is turned on
- Electricity that flows on copper cables coated with insulators
- When using a grill tool
- Weld or men soldiers
Therefore, many of conductor tools are made to help human fulfill their needs and support their activities. For detail information, you can read it on article the use of conductors in life
Conductive Heat Transfer Process
Not all materials are conductor and each material has different values. The greater the conductivity, the better the heat. Usually, metal has good conductivity to transfer heat. The following is the data of thermal conductivity values of various substances.
Value of Thermal Conductivity (K) Various Materials At 0 ° C
| Material | W/m x °C | Btu/h x ft x °F |
| Metal | ||
| Silver (pure) | 410 | 237 |
| Copper (pure) | 385 | 223 |
| Aluminum (pure) | 202 | 117 |
| Nickel (pure) | 93 | 54 |
| Iron (pure) | 73 | 42 |
| Carbon steel, 1% C | 43 | 25 |
| Lead (pure) | 35 | 20,3 |
| Chrome-nickel steel | 16,3 | 9,4 |
| (18% Cr, 8% Ni) | ||
| Non Metal | ||
| Quartz (parallel axis) | 41,6 | 24 |
| Magnesit | 4,15 | 2,4 |
| Marmar | 2,08-2,94 | 1,2-1,7 |
| Sandstone | 1,83 | 1,06 |
| Glass, window | 0,78 | 0,45 |
| Maple or oak wood | 0,17 | 0,096 |
| Sawdust | 0,059 | 0,034 |
| Glass wool | 0,038 | 0,022 |
| Liquid | ||
| Mercury | 8,21 | 4,74 |
| Water | 0,556 | 0,327 |
| Ammonia | 0,540 | 0,312 |
| Lumas Oil, SAE 50 | 0,147 | 0,085 |
| Freon 12,CCl2 F2 | 0,073 | 0,042 |
| Gas | ||
| Hydrogen | 0,175 | 0,101 |
| Helium | 0,141 | 0,081 |
| Air | 0,024 | 0,0139 |
| Water vapor (saturated) | 0,0206 | 0,0119 |
| Carbon dioxide | 0,0146 | 0,00844 |
The heat transfer of conduction is caused by the activity of the molecule which causes the heat energy to diffuse. Each object has particles that form the object. Generally, the solid state has a solid composite particles that has a close distance between each particle.
So when there are objects that have a high conductivity that is heated and was taken near the objects, the end of the conductor object which is close to the heat energy will collide and the particles next to it, also the energy will be moved to the object that is close.
You may also read about:
- List of the Categories of Essential Biochemical Compounds
- Common Chemical Oxidizing Agents
- Sulphur Dioxide Uses
That’s the explanation of the heat transfer through conduction example in daily life. Hopefully it can help you to learn more.