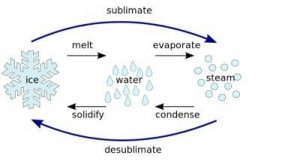
 Gas is one form of matter which have free particles and irregular configuration. While solid is a form of matter with regular configuration of its particles. Transformation of matter can occur due to temperature changes that occur in objects. Transformation of matter from gas to solid is also called disposition or known as crystallizing.
Gas is one form of matter which have free particles and irregular configuration. While solid is a form of matter with regular configuration of its particles. Transformation of matter can occur due to temperature changes that occur in objects. Transformation of matter from gas to solid is also called disposition or known as crystallizing.
Such transformation process can occur naturally or artificially. Examples of transformation of gas to solid naturally such as when water vapor turns into dense snowflakes. While artificial instance is not deliberately done such as pollution generated from motor vehicles in the exhaust often has dried black solid soot due to the exhaust of gas engine.
But in addition, there is also a useful process of artificial crystallization which is the process of making dry ice from carbon dioxide, manufacture of ammonium sulfate and ammonium nitrate.
Sample of Transformation of Gas into Solid
Here are the examples of transformation of gas into solid solution:
1. Formation process of snow from water vapor
Water vapor that accumulates in the atmosphere will reach the point of condensation in which the gas temperature turns into solid. Thus it forms clouds with a mass smaller than air. Rupture of water vapor is due to maximum capacity of clouds. If the rupture of water particle sent down to the earth is called rain, it is one of the natural phenomena occurring in the troposphere. And if its temperature is below 0°C, it will fall down as snow.
2. Black soot on motor exhaust
Burning is a chemical reaction of fuel with oxygen. In combustion of diesel motor, diesel fuel mix is put into the combustion chamber with air adiabatically. The smoke emitted by a diesel motor is particulate in gas waste containing PAHs and soot.
The black gas is a sign of burning failure. We need to be concerned for black soot as it contains carcinogens that can lead to cancer in humans. The formation of soot can be decreased by reducing the engine load. The low machine loads will also result in a little soot.
3. Process of Making Dry Ice from Carbon Dioxide
Charles Thilorier was the first person who observed the presence of solid CO2 in 1835 when he opened a pressurized liquid CO2 container. CO2 will develop a solid form without a liquid phase at a pressure of 5.1 atm and a temperature of about -79°C. The solid form is known as dry ice.
Its function is to provide smoke effects on the stage performance. The smoke has a density between 1,2 and 1,6 kg/dm3 which is larger than the air density that it will not rise up. We should avoid direct contact or use gloves as they may cause cold burns. Moreover, we shall use it in good ventilation as otherwise it will cause respiratory problems. Also read: Carbon Uses in Daily Life
4. Preparation of Ammonium Nitrate Material
Ammonium Nitrate is an inorganic compound. This compound is used as explosive raw materials and raw material for manufacture of nitrogen fertilizers. There is a form of powder and granules (prilled).
Type of production process of making Ammonium Nitrate:
- Grainer: The longest and rarely used process is by means of nitrate ammonium solution concentrated to 98% at temperature of 305-310°. Crystallization is performed by stirring evenly fo form the crystals containing 0.1% of moisture weight carried out on Graining Kettle. This process is high-priced and hazardous.
- Prilling: Ammonia gas and Nitric Acid are reacted with a neutralization reaction in an exothermic reactor to produce steam. The maximum temperature is 200°C with output reactor exchanger concentration of 85% of The solution is concentrated by falling evaporator up to 99.8% of weight (for explosive substance industry). Then it is pumped to prilling tower and the formed ammonium nitrate prill is cooled and sieved to obtain a homogeneous grain coated with Calcium Tri Phosphat and the last stages is packing.
- Stengel: Ammonia gas and nitric acid are heated continuously from above vertical packed reactor (maximum temperature of 200°C). The solution is then fed into the cyclon separator along with the reactor. Output of separator product is the melted Ammonium Nitrate with water content of 0.2% weight with the melting temperature of 200°. Then through Prilling tower, the melt is dropped down that it forms small balls (prill) or turns into flakes by cooling it on the drum or belt. The debris is sieved and coated with Calcium Tri Phosphate in a coating drum that it does not clot when stored in storage/zak.
- Uhde: The process of reacting ammonia gas and nitric acid in the bubbling reactor by neutralizing reaction at a pressure of 4-5 bar with a temperature close to 200° The reactor is inserted into the flash drum when the solution comes out and concentrated by pumping it into the evaporator. The steam coming out of the evaporator is partially utilized as a heater and partly fed to the absorber as an ammonia gas absorber. Afterwards it goes into prilling tower. Ammonium nitrate flakes are cooled and screened according to the desired form. It is most used and popular as it is affordable with low investment.
That’s the complete examples of transformation of gas into solid solution.
5. Preparation of Ammonium Sulphate Material ((NH4) 2SO4)
Ammonium sulfate is an anionic salt with 21% nitrogen content (ammonium cation) and 24% sulphur (anion sulphate). It is obtained from the process of ammonia treatment with sulfuric acid from oven-coke by chemical reaction: 2 NH3 + H2SO4 → (NH4) 2SO4
The process is by mixing ammonia gas and water vapor into a reactor containing an ammonium sulfate solution of about 2-4% of free sulfuric acid solution sample at 60°C. In order to maintain the acidity, it needs to be added with concentrated sulfuric acid. The dry powder of ammonium sulfate may be formed by spraying sulfuric acid into a reaction container containing ammonia gas. The heat of its reaction evaporates all the water in the system that it eventually forms flour-like salt. Also read: Strong Bases and Weak Bases Examples in Daily Life
Stages of Crystallization Process
Crystallization process in the industrial scale is by the method of modifying pH of the system. Other than that, it can also be chemical or evaporative reaction. The main stages in crystallization process are:
- Nucleation: The molecules dispersed in solution or gas phase will form a nanometer-sized (very small) crystalline seed-shaped bond, and in order to stabilize the seedlings it requires to increase the size of crystalline seedlings.
- Crystalline Development: in order to form a large and stabilized crystal seed it needs to be conditioned by controlling temperature, saturation, pressure and so on.
Thus, hereby discussion of gas samples into solid. Hopefully it can give you more insights and useful advantage about the examples of transformation of gas into solid solution!
Here are m ore to learn: