 pH is an indicator that indicates whether the solution is acidic, neutral or alkaline. pH can also be interpreted as the concentration of hydrogen ions contained in the solution. The pH can also be translated as a negative logarithm value of the hydrogen ion concentration.
pH is an indicator that indicates whether the solution is acidic, neutral or alkaline. pH can also be interpreted as the concentration of hydrogen ions contained in the solution. The pH can also be translated as a negative logarithm value of the hydrogen ion concentration.
Is pH very important in human life especially in water? What if the water is too acidic? Or too alkaline or neutral? What pH is safe for humans? Here will be discussed in more detail about the measuring pH value of water.
Before getting into the main material, about the pH in water we need to know what is water? Water is the source of life for humans, without water there will be no life on earth. Therefore water is needed by human life. However, there is one important point that not all water can be consumed by humans. Because, there is water that has toxins in it, dirty water and so on. One indicator that indicates that water is feasible or unfit for consumption is pH.
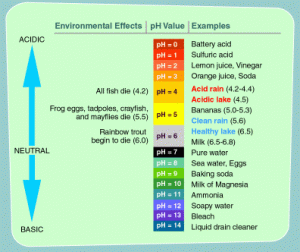
pH has a value from 0 – 14. The value is in accordance with international standards where pH 1 – 6 is said to be acidic solution, pH 7 is neutral and pH 8-14 is a base. How can water have a variety of pH?
First, we see that water is a universal solvent, so that if there are substances that enter into the water it can affect the water itself. For example, have you ever seen a fence in the yard of a house made of iron over time will rust when hit by rain water? Yep. In addition to the effects of the water itself that can rust with the formation of Fe3O4, the pH in the rainwater itself has a pH value about 5-6.
This is due to the high levels of CO2, S, CO and so on that comes from vehicle fumes, and factory pollutants. As well as the abundance of trees being felled causes the high levels of these acids into the air causing acid rain. In addition to the negative effects of acidic pH, there is an advantage if the water has an acid content. Among them, there are some plants that require acid solution, for growth, then on an industrial scale requires a slightly acidic pH to make the bacteria die, make the shampoo feel sore in the eyes.
It has been described how the pH of water affects the life of humans. Now, how to measure the pH of the water itself? Simply put, there is a device called pH meter that can measure the pH of the water instantly, or it can also use the universal indicator and match it with the available table or use red or blue litmus paper which is marked with the color change on one of the papers.
But a fairly efficient tool to use is to use pH meter, because pH meters can be used multiple times and can be used to know the pH value directly from the monitor screen. Whereas if using universal indicator and litmus paper only for use with laboratory scale. Because the exact number of concentrations of the solution is not very visible and can not be used repeatedly or in the long run. To measure the pH of the solution, we can use a calculation made mathematically by Soren Peter Lauritz with the formula :
pH = – Log [H +]
Where [H +] is the concentration of hydrogen ions in molarity or mol / L. The value of pH itself depends on the concentration of [H +] and [OH-] ions. When the value of [H +] is greater than [OH-] the solution is said to be the opposite acid solution examples, if the concentration of [H +] is lower than the concentration of [OH-] then the solution is said to be a base solution. (Read also examples of Strong and Weak Bases solution)
Problem examples
A solution is known to have a concentration of [H +] of 1 x 10-6 mol / L what is the pH of the concentration value?
Answer:
pH = – Log [H +]
pH = – log (1 x 10-6)
pH = – (log 1 + log 10-6)
pH = – (0 + (-6))
pH = 6
So the solution has a pH value of 6. Then, because of the concentration of hydrogen ions and the constant concentration of hydroxide in a solution, one can be quantitatively or qualitatively calculated. Therefore, when measuring the pH of a solution, usually when we calculate hydrogen ions or concentration [H +] we indirectly measure the [OH-] level as well
[H +] [OH-] = 10-14
Answer:
pH = – Log [H +]
pH = – log (1 x 10-6)
pH = – (log 1 + log 10-6)
pH = – (0 + (-6))
pH = 6
So the solution has a pH value of 6
Then, because of the concentration of hydrogen ions and the constant concentration of hydroxide in a solution, one can be quantitatively or qualitatively calculated. Therefore, when measuring the pH of a solution, usually when we calculate hydrogen ions or concentration [H +] we indirectly measure the [OH-] level as well
[H +] [OH-] = 10-14
Read also :
From the above explanation it can be concluded that measure pH of water is very important. Because it gives a direct impact on human life. the pH that humans need to consume is 6.5 – 8.5 whereas the pH above or below of the value is not necessarily said to be bad because there are some industries that need water in acid or alkaline conditions in running their business. (Read also Acid in Water Reaction)

