 Gas is a form of particles that move freely without being seen. The smallest part of the particle is called the atom. It turns out that the atom itself is composed of electrons (negatively charged particles) that surround the nucleus which has protons (positive mutant particles) and neutrons in it.
Gas is a form of particles that move freely without being seen. The smallest part of the particle is called the atom. It turns out that the atom itself is composed of electrons (negatively charged particles) that surround the nucleus which has protons (positive mutant particles) and neutrons in it.
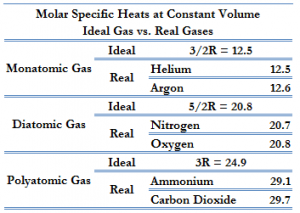
Gas atoms can be monoatomic, diatomic and poliatomic. The following examples of monatomic and diatomic gases.
Monoatomic Gas Example
Monoatomic gas as the name implies mono means single is a feature of simple ions which are only one single atom. A stable monoatomic gas for example is a noble gas. Besides the noble gas monoatomic gas is unstable. The noble gases in the periodic system are class VIIIA. The least gaseous gas in nature. This element is largely unreactive because the electron configuration is unstable. Here are 6 examples of monoatomic gases, explanations and their functions:
1. Helium (He)
The nature of helium gas is inert or light, non-flammable and at low boiling point is not reactive. Its function:
- Air balloon filling
- Replacement of nitrogen to be used by divers for diving on the ocean floor to see the ecology of tropical seas
- As an oxygen solvent in the oxygen tube for medical at the hospital
- As metal coil coolant (liquid helium) for body scanner due to steam pressure drop.
Here is it: Helium Uses in The World
2. Neon (Ne)
Is noble gas that is not reactive. Gas in low pressure tube, if it is given electric voltage, it will produce red light with high intensity.
Its function:
- Fill neon lights neither lights nor neon boxes for advertisements
- Coolant
- Lightning rod
- Television tube filler
3. Argon (Ar)
The argon noble gas is also equally inert (lightweight) and non-reative, so it is safe to use for:
- As illumination of incandescent light bulbs
- Inert atmosphere of titanium and stainless steel welding
- In semi conductor industry as synthetic single crystal / germanium
4. Krypton (Cr)
Krypton properties that benefit us is when given the electrical charge it will produce white light with high intensity. So it can be used for:
- Airplane lamp lighter filler
- Low-pressure floutensen filling lamp
- As a flash for high speed photography
5. Xenon (Xe)
Just like krypton if it given an electric charge will produce a very bright white light beyond krypton. Therefore the price is very expensive. Its function is:
For photographic flash lamps
- Charger of some car lights
- As an anesthetic (anesthetic)
- For bacteria killer (bactericide)
- Making electron tube
6. Radon (Rn)
Radon is the most radioactive gas than any other monoatomic gas element. This gas serves to:
- For cancer radiotherapy (radiotherapy therapy)
- Indicators of radioactive mineral presence (uranium, rocks and building materials)
- As an earthquake warning system that is when the earth plate moves then radon levels will change to be biased
Examples of other monoatomic gases are the aniom which are Cl-, F-, I-, but they are unstable.
Diatomic Gas Example
In Greek, di- means two, the diatomic gas is a molecule formed by two atomic elements. Generally a covalent bond and its content is widely present in nature. There are two kinds of diatomic gases such as Nitrogen (N2), Oxygen (O2), Hydrogen (H2), etc. Another heteronuclear diatom gas are compound of NO, CO, HCl. Here are four examples of diatomic gases and their explanations and functions:
Examples of other monoatomic gases are the aniom which are Cl-, F-, I-, but they are unstable.
1. Nitrogen (N2)
Nitrogen is the most abundant element in the universe. Its presence on earth is about 78% in the Atmosphere. The nature of nitrogen is less reactive so it is difficult to react with other elements, inert (lightweight) and easily evaporate. With those characteristics of the nitrogen, it can be utilized as follows:
- As a cooler
- Helps medical dermatologists deal to burns warts and benign skin
- Inhibition of bacterial growth so that food will be durable from decay.
Here is it:
2. Oxygen (O2)
Oxygen is an abundant gas after nitrogen. Its existence fills the earth’s atmosphere around 20.9%. Colorless and odorless, yet everyone can feel through the breath. The process of breathing or respiration requires oxygen. Its function is vital to human survival in this world. Oxygen is very reactive and easy to react with other elements. Here’s the oxygen function:
- The process of respiration for humans
- Prevent the growth of anaerobic cells
- Helps the circulatory system
- Helps to fly an airplane
- As an explosive
- For welding materials (metal connecting)
- For medical stocks stored in oxygen-relief tubes for asthmatics
- For oxygen for the diver while diving in the ocean
3. Hydrogen (H2)
Hydrogen is the lightest element in the world (inert). The word “hydrogen” is taken from two words in Greek, hydro, which means water and genes which means to form. It is highly flammable, colorless, odorless and single-valent. Its function are :
- For the hydrogenesis reaction of various organic compounds
- As a reducer of some compounds
- Basic ingredients of fertilizer made with nitrogen
- As transportation fuelIn
- dicator of gas leakage
Here is it: Hydrogen Uses
4. Nitrogen Monoxide Compound (NO)
Nitrogen Monoxide is a heteronuclear diatom molecule composed of two different elements, namely Nitrogen and Oxygen. Basically these compounds are free radicals that come from the result of burning of fossil fuels and is naturally generated from lightning when releasing electricity. Its benefits are :
- Gas that can be slightly diffuse and bioactive molecules
- The basic ingredients of fertilizer maker
- Detect radical on the polymer.
- Helps improve inter-cell communication in the cardiovascular system (blood vessels and heart).
- Helps treatment of pulmonary hypertension in infants who are delivered at low concentrations.
- Sufficient body-level NO to maintain the liver from ischemic damage due to sepsis. because NO can be produced by neurons during the human brain without causing poisoning effects,
Thus explanation of examples of monatomic and diatomic gases May be a useful knowledge for all of us.
Read also

